Morphology
P.v morphology
| Plasmodium vivax:Blood Stage Parasites
Thin Blood Smears Illustrations from: Coatney GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda, 1971. |
|
|
| Fig. 1: Normal red cell; Figs. 2-6: Young trophozoites (ring stage parasites); Figs. 7-18: Trophozoites; Figs. 19-27: Schizonts; Figs. 28 and 29: Macrogametocytes (female); Fig. 30: Microgametocyte (male) |
|
|
| Plasmodium vivax:Blood Stage Parasites
Thick Blood Smears Illustrations from: Wilcox A. Manual for the Microscopical Diagnosis of Malaria in Man. U.S. Department of Health, Education and Welfare, Washington, 1960. |
P.f morphology
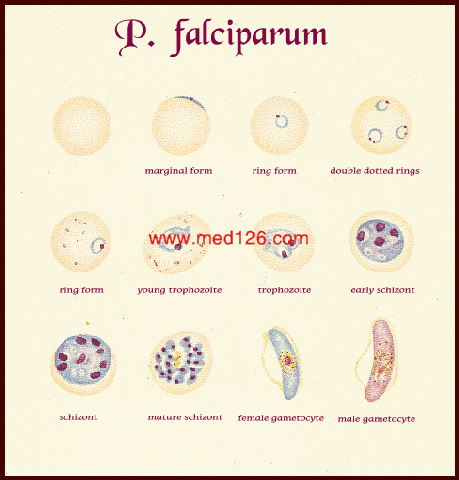
| Plasmodium falciparum:Blood Stage Parasites
Thin Blood Smears Illustrations from: Coatney GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda, 1971. |
|
|
| Fig. 1: Normal red cell; Figs. 2-18: Trophozoites (among these, Figs. 2-10 correspond to ring-stage trophozoites); Figs. 19-26: Schizonts (Fig. 26 is a ruptured schizont); Figs. 27, 28: Mature macrogametocytes (female); Figs. 29, 30: Mature microgametocytes (male). |
|
Plasmodium falciparum:Blood Stage Parasites Thick Blood Smears Illustrations from: Wilcox A. Manual for the Microscopical Diagnosis of Malaria in Man. U.S. Department of Health, Education and Welfare, Washington, 1960. |
|
|
Ring forms (early trophozoites)
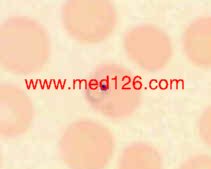
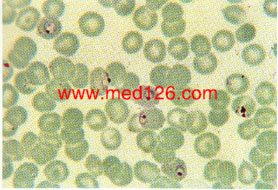
P. vivax thin smear, showing early trophozoites.
The infected red cells are enlarged and show some stippling. Giemsa. ×1000. Enlarged by 5.4.
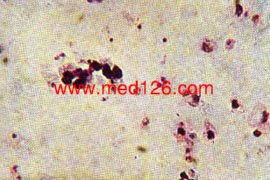
P. vivax thick smear, early trophozoites.
The red cells are lysed. Giemsa. ×1000. Enlarged by 5.4.
Late trophozoites
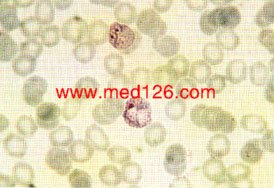
P.vivax thin smear.
Showing late trophozoites (amoeboid stage). The infected red cells are enlarged and show marked stippling. Giemsa. ×1000. Enlarged by 5.4.
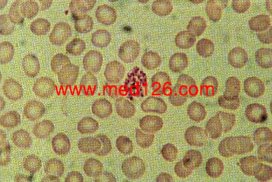
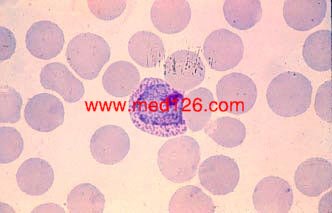
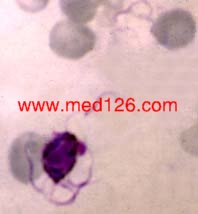
Schizonts
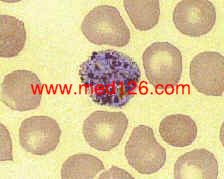
P. vivax thin smear.
A mature schizont about to rupture. A clump of malarial pigment can be seen in the center. Giemsa. ×1000. Enlarged by 5.4.
P. vivax thin smear.
Merozoites lying free. Malarial pigment is seen as a clump on one side. Giemsa. ×1000. Enlarged by 5.4.
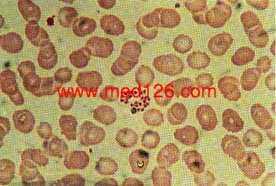
Male gametocytes
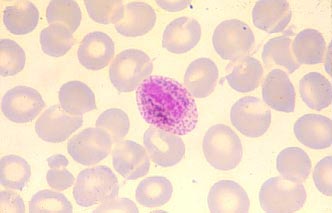
Female gametocytes
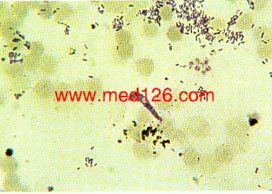
Exflagellation
Zygote(No pic available now)
Ookinete
|
|
|
 | |
|
Ookinete, from the midgut of an infected mosquito. Giemsa. ×800. Enlarged by 5.4. | |
Oocyst
|
|
|
|
| |
| Oocysts of P. falciparum in midgut of an infected mosquito. Oocysts appear as circular bodies. Fresh preparation. ×100. Enlarged by 5.4. | |
Sporozoite
 |
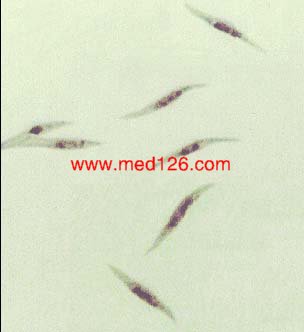 |
Exo-erythrocytic forms(EEF)
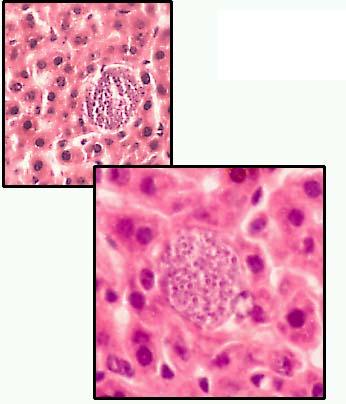
红外期裂殖体
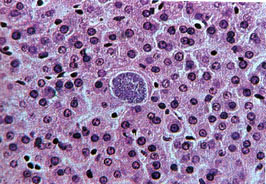
Pre-erythrocytic schizont in liver.
These mature in 6-14 days’ time liberation merozoites into the blood stream. Giemsa-colophonium. ×400. Enlarged by 5.4.
Pathogenesis
1. Incubation period: P.v. 14-17 days P.f. 8-12 days
2. An attack occurs because of the sudden liberation of merozoites, malarial pigment and RBC debris into the blood stream.
Three stages of each paroxysm
(1) The cold stage (chill) lasting for 30min-1hr
(2) The hot stage(fever) lasting for 1-4hr (P.v. P.f. and P.o. once every other day; P.m. once every 2 days. )
(3) Sweating stage 1-2hr
4. Recrudescence and Relapse
P.f and P.m. have only recrudescence, but, P.v. and P.o. both have relaps and recrudescence.
remnant of parasites in RBC result in recrudescence
hypnozoites cause relapse
5. Anemia; splenomegalia and fatal malaria-cerebral malaria caused by P.f. (small vessels are plugged)
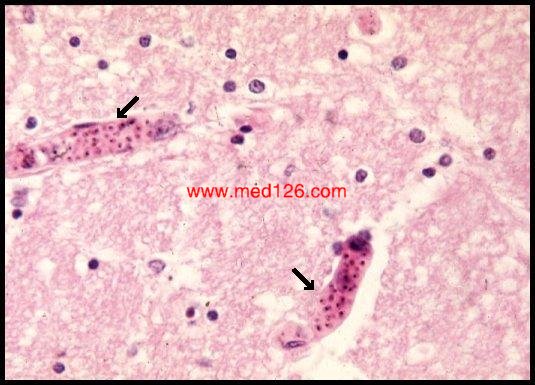
Diagnosis
A. Clinical symptoms and history
B. Microscopic examination of blood.
1 Thin film and Thick(Giemsa's stain)
To master the morphology of parasites and changes of infected red cells
2 P.f.: Only Ring forms and gametocytes can be found in blood film.
C. Other methods: Immunologic/Biochemical/Molecular diagnosis.
Laboratory Diagnosis
There are four species of malaria which commonly infect man, P.falciparum, P.vivax, P.malariae and P.ovale. The most important of these is P.falciparum because it can be rapidly fatal. P.vivax is the most commonly seen species. P.malariae is present in Africa, South America and in one area of New Guinea. P. ovale is relatively unusual outside Africa although some cases are now being identified in other regions(eg. Southern States of India).
A number of new techniques based on the "dipstick" format, have recently become available for the diagnosis of malaria. These include the ICT-Malaria Pf, OptiMALr and the Determine kits. The methods are based on the principle of the detection of plasmodial histidine rich protein-2 (HRP-2) or parasite-specific lactate dehydrogenase (pLDH) which is present in P. falciparum infections, with a number of reports claiming sensitivities and specificities approaching 100%. Some of these "dipstick" methods have been extended to include screening for other forms of malaria but to date results have not been quite so impressive.
In this laboratory we have found these kits to be very useful screening or confirmatory tests, especially when there is difficulty in identifying scanty ring forms in blood films. They are particularly useful out of hours when more junior, less experienced staff are likely to be on duty. However we would like to emphasise, that we regard these methods as additional to the long established method of examining thick and thin blood films (outlined below), which is still regarded as the "gold standard", NOT as replacement methods.
Examination of a thick blood film should be the first step, if parasites are seen then the species should be confirmed by the examination of a thin film. Blood is best collected when the patient's temperature is rising.
Preparation of thick and thin blood films :-
Thick films:- place a drop of blood in the middle of a clean microscope slide and with the corner of a second slide spread the drop until it is about the size of a five cent coin. The thickness should be such that it is just possible to see news print through it. Thin films are made in the standard manner. Allow the films to dry, do not leave on the bench in a laboratory which is not fly proofed otherwise the film will be eaten.

When the films are dry, fix and stain the thin films in the conventional manner but be careful about the pH of the stain, a slightly alkaline stain is recommended (pH 7.2) as an acid stain may fail to show the parasites. When only a few thick films are to be stained it is best to use dilute Giemsa stain (1/20), using a staining jar so that the film is in an upright position, this will allow any debris to fall to the bottom of the jar. Do not fix the sample prior to staining. Stain for about 30 minutes, wash gently with clean water and allow to dry. If available use a positive control. When a large number of thick films require staininq, Field's stain is preferred because it is very quick. Field's stain comprises two solutions; a polychrome methylene blue (A) and eosin (B). The solutions are kept in covered staining jars.
If films are old or too thick the red cells may not lyse completely in the brief staining time. If this is likely dip the film in clean water for a few seconds or until the haemoglobin has dispersed before staining. Instructions for preparing Field's stain can be found in many laboratory text books.
Under the microscope examine the thick film first, using an oil immersion or high dry lens to determine if parasites are present. Be aware of the patient's platelet and leucocyte counts. Malaria is usually associated with a normal or reduced leucocyte numbers. A leucocytosis is only found in terminal cases. Platelet numbers are moderately or markedly reduced in some 80% of patients with malaria. Parasites may appear distorted if the patient has been treated or has had inadequate prophylaxis.
Mixed infections are not uncommon.
The illustrations show the characteristics of the four species.
 |
|---|
Diagnostic points:-
|
 |
|---|
Diagnostic points:-
|
 |
|---|
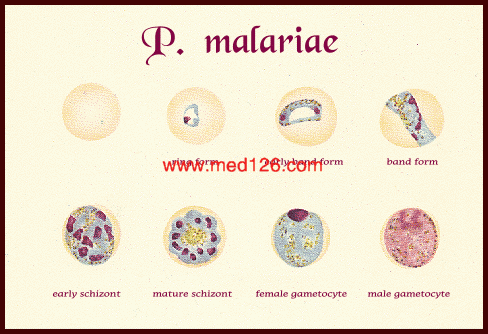
Diagnostic points :-
|
 |
|---|
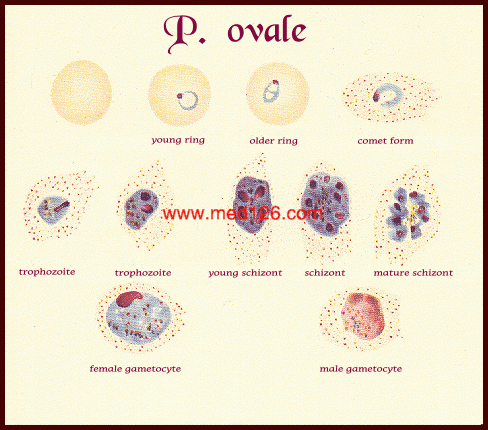
Diagnostic points :-
|
| Plasmodium species | Stages found in blood | Appearance of Erythrocyte (RBC) | Appearance of Parasite |
| P. falciparum | Ring | normal; multiple infection of RBC more common than in other species |
delicate cytoplasm; 1-2 small chromatin dots; occasional appliqué (accollé forms) |
| Trophozoite | normal; rarely, Maurer's clefts (under certain staining conditions) | seldom seen in peripheral blood; compact cytoplasm; dark pigment | |
| Schizont | normal; rarely, Maurer's clefts (under certain staining conditions) | seldom seen in peripheral blood; mature = 8-24 small merozoites; dark pigment, clumped in one mass | |
| Gametocyte | distorted by parasite | crescent or sausage shape; chromatin in a single mass (macrogametocyte) or diffuse (microgametocyte); dark pigment mass | |
| P. vivax | Ring | normal to 11/4 X, round; occasionally fine Schüffner's dots; multiple infection of RBC not uncommon | large cytoplasm with occasional pseudopods; large chromatin dot |
| Trophozoite | enlarged 11/2-2 X; may be distorted; fine Schüffner's dots | large ameboid cytoplasm; large chromatin; fine, yellowish-brown pigment | |
| Schizont | enlarged 11/2-2 X; may be distorted; fine Schüffner's dots | large, may almost fill RBC; mature = 12-24 merozoites; yellowish-brown, coalesced pigment | |
| Gametocyte | enlarged 11/2-2 X; may be distorted; fine Schüffner's dots | round to oval; compact; may almost fill RBC; chromatin compact, eccentric (macrogametocyte) or diffuse (microgametocyte); scattered brown pigment | |
| P. ovale | Ring | normal to 11/4 X, round to oval; occasionally Schüffner's dots; occasionally fimbriated; multiple infection of RBC not uncommon | sturdy cytoplasm; large chromatin |
| Trophozoite | normal to 11/4 X; round to oval; some fimbriated; Schüffner's dots | compact with large chromatin; dark-brown pigment | |
| Schizont | normal to 11/4 X, round to oval, some fimbriated, Schüffner's dots | mature = 6-14 merozoites with large nuclei, clustered around mass of dark-brown pigment | |
| Gametocyte | normal to 11/4 X; round to oval, some fimbriated; Schüffner'dots | round to oval; compact; may almost fill RBC; chromatin compact, eccentric (macrogametocyte) or more diffuse (microgametocyte); scattered brown pigment | |
| P. malariae | Ring | normal to 3/4 X | sturdy cytoplasm; large chromatin |
| Trophozoite | normal to 3/4 X; rarely, Ziemann's stippling (under certain staining conditions) | compact cytoplasm; large chromatin; occasional band forms; coarse, dark-brown pigment | |
| Schizont | normal to 3/4 X; rarely, Ziemann's stippling (under certain staining conditions) | mature = 6-12 merozoites with large nuclei, clustered around mass of coarse, dark-brown pigment; occasional rosettes | |
| Gametocyte | normal to 3/4 X; rarely, Ziemann's stippling (under certain staining conditions) | round to oval; compact; may almost fill RBC; chromatin compact, eccentric (macrogametocyte) or more diffuse (microgametocyte); scattered brown pigment |
Treatment
1. Three principles:
Malaria Treatment
P. falciparum.
This species was originally sensitive to chloroquine, however, strains resistant to this and other antimalarial drugs are now commonplace. Because the parasite is able to multiply very rapidly and sequester within the microvasculature, a life threatening illness may develop in a very short space of time.
Uncomplicated malaria (where patients can take oral therapy) can be treated with one of three regimens: